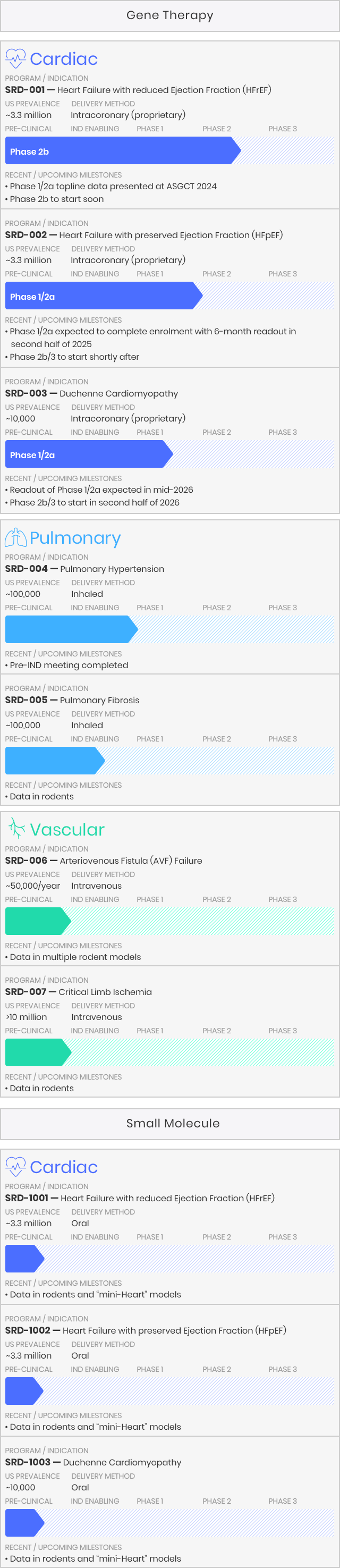
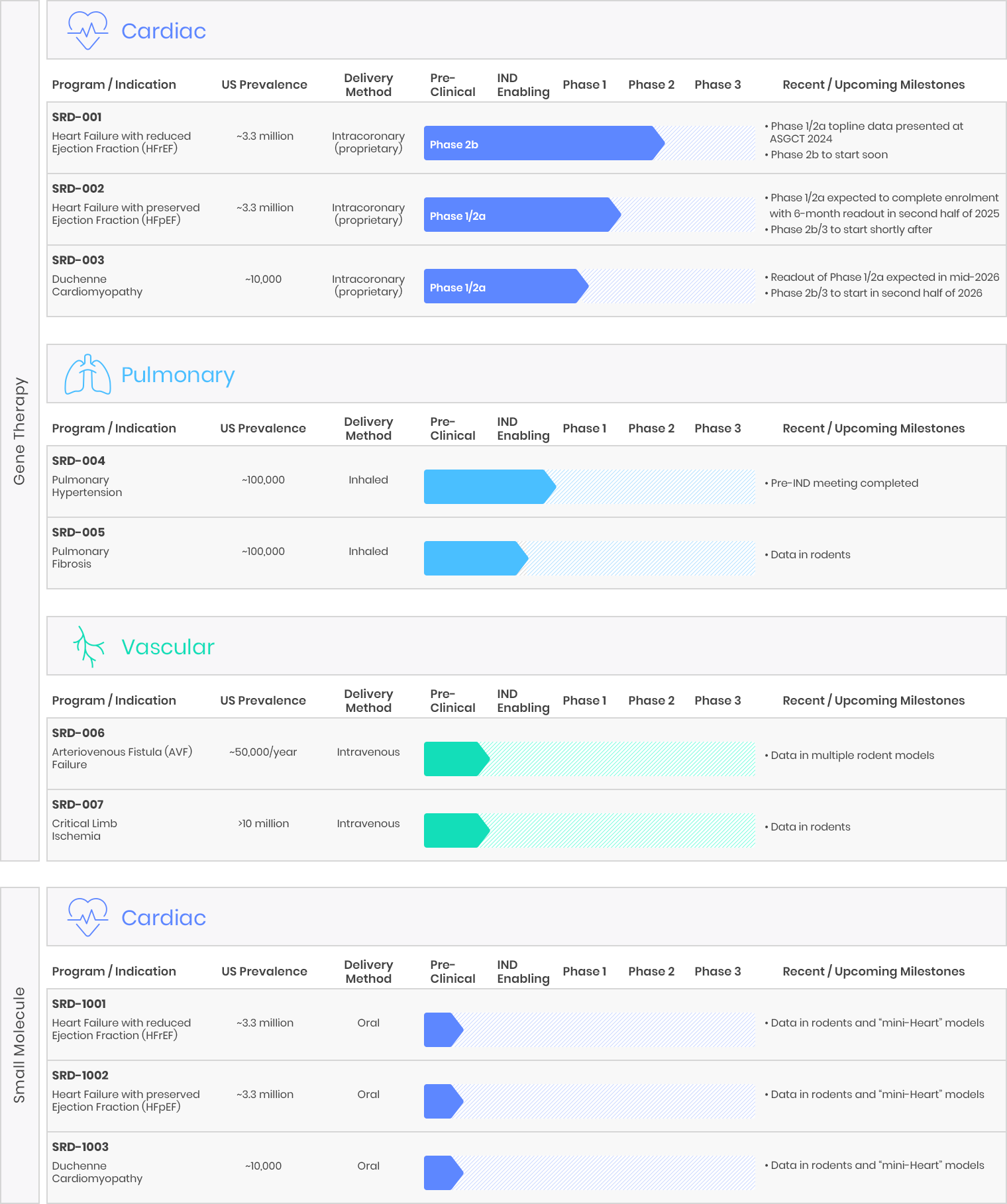
Medera is advancing a pipeline of disease-modifying gene therapies and small molecule candidates developed by our team. These candidates are designed to target a range of prevalent and rare cardiac, pulmonary and vascular diseases that have significant unmet needs and no approved treatments that address the underlying molecular cause of the disease. Our pipeline includes programs that have emerged from our internal discovery and validation, as well as programs originally based on scientific research from leading academic institutions. We retain exclusive worldwide development and commercialization rights to all our product candidates and programs.
To maximize therapeutic impact, we strategically target serious, life-threatening difficult-to-treat or incurable diseases currently without any approved disease-modifying therapeutics, thereby representing significant unmet medical needs. By adopting scientifically-based and technologically-driven approaches to each of our targeted disease indications, we aspire to develop Best-In-Class medicines for the benefit of patients. Furthermore, our proprietary intracoronary delivery method substantially reduces side effects and the cost-of-goods by as much as 100 times (versus conventional systemic methods such as IV infusion).
As appropriate, we apply for accelerated regulatory pathways for approval with the US FDA. For example, Fast Track Designation (FTD) and Orphan Drug Designation (ODD) have been obtained for our HFpEF and DMD-CM programs, respectively. We advanced our lead candidates into the clinic by efficiently utilizing our preclinical models, inclusive of our unique human mini-Heart technology platform.