Our Divisions
Medera’s corporate divisions include Novoheart, focused on pre-clinical disease modeling and drug discovery, and Sardocor, focused on clinical development of novel gene and cell therapies. Our comprehensive and integrated capabilities allow us to efficiently and effectively operate throughout the entire process of therapeutic discovery and development, and to advance our lead candidates along expedited regulatory pathways for the benefits of patients.
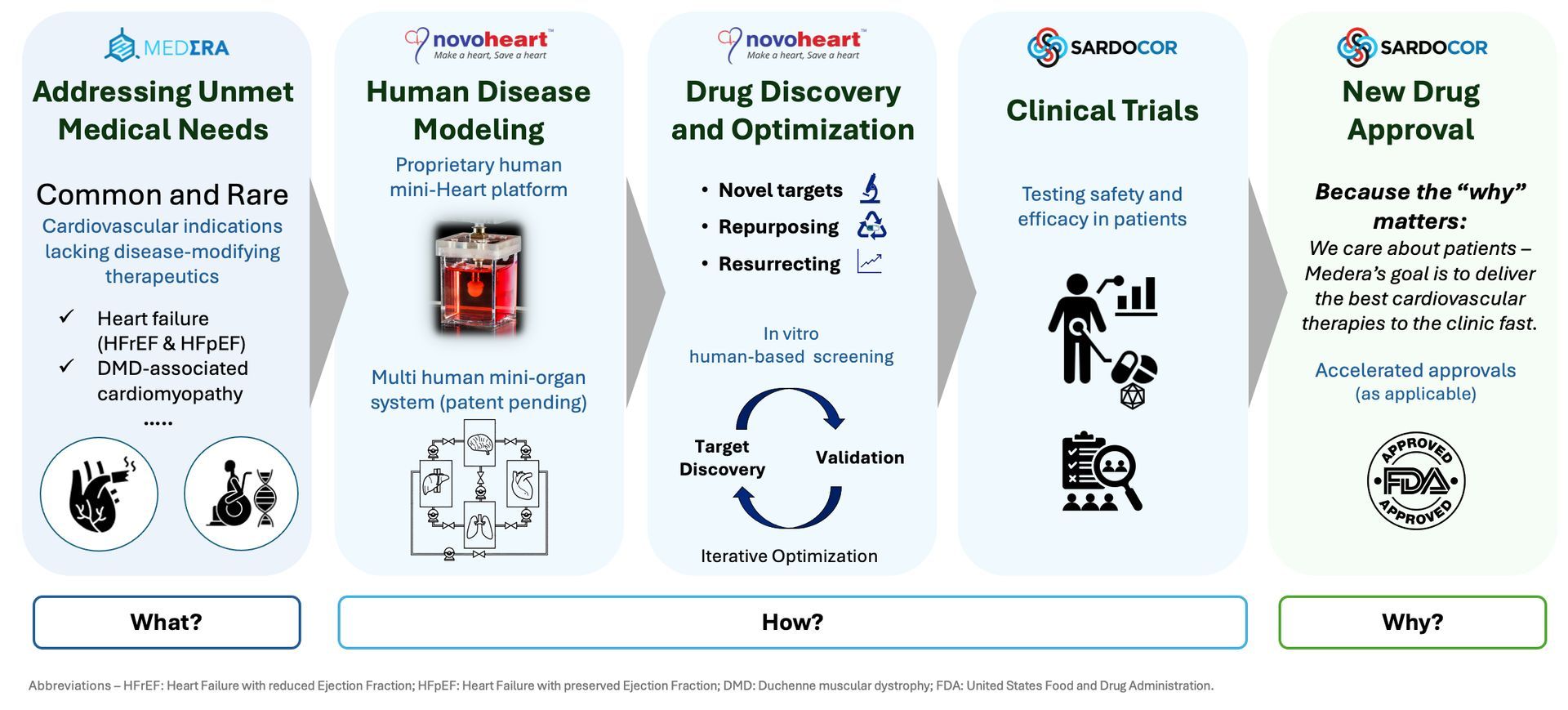
Founded in 2014, Medera’s Novoheart has an extensive corporate history in commercializing bioengineered human cardiac tissues for disease modeling and drug screening. Our founders were among the first biomedical engineers who established the field back in the 1990s. Novoheart offers the industry’s most sophisticated and comprehensive human mini-Heart platform, including Novoheart's unique Human-Heart-in-a-Jar, as vitro models for generating human-specific preclinical drug safety, efficacy, and dosing data to help accelerate the drug discovery and FDA regulatory processes.
Our objective is to create, expand, validate and optimize Medera’s therapeutic pipeline. In accordance with the FDA Modernization Act 2.0, the Novoheart platform has facilitated and accelerated the development of our lead therapeutic candidates (under the “Sardocor” brand) currently being evaluated in clinical trials for three cardiac disease indications.
Medera’s Sardocor was founded by scientific visionaries who started the field of Cardiac Gene Therapy over three decades ago at Massachusetts General Hospital and Harvard Medical School. Sardocor is dedicated to the clinical development of novel therapies for patients with incurable cardiovascular diseases.
We have been granted Investigational New Drug (IND) clearances by the US Food and Drug Administration (FDA) for three ongoing adeno-associated virus (AAV)-based cardiac gene therapy clinical trials for heart failure with preserved ejection fraction (HFpEF), Duchenne muscular dystrophy-associated cardiomyopathy (DMD-CM), and heart failure with reduced ejection fraction (HFrEF). We also have a pipeline of preclinical gene therapy and small molecule candidates for a range of cardiac, pulmonary and vascular diseases.
Using our proprietary intracoronary infusion methodology, Sardocor's gene therapy candidates are directly delivered via the blood vessels of the heart to the cardiac ventricular muscle cells as an out-patient procedure. This minimally invasive procedure allows the delivery of the optimal and least amount of drug product (up to 100 times less viral titers compared to conventional intravenous (IV) methods for systemically administering gene therapy) to achieve efficient transduction and improved efficacy while avoiding the side effects typically seen with systemic delivery of very large doses. Furthermore, our data suggest that achieving therapeutic effect will not depend
on viral vector serotype/tropism or require any form of immunosuppression. Overall, our unique patented method, exclusively owned by Medera’s Sardocor, has the potential to substantially improve drug specificity and safety, while reducing costs.




